China's State Council issued the 12th Five-Year Development Plan (2011-2015) for Pharmaceutical Safety Standard Monday, to raise industry standards and improve safety supervision.
The plan, formulated by the National Development and Reform Commission, said that the government is to raise the quality standards of 6,500 Chinese-made medicines, including 2,500 synthetic drugs, 2,800 processed Chinese medicines, 200 bio-pharmaceuticals, 350 medical herbs and 650 traditional Chinese medical decoctions.
China will also improve the requirements for 239 drug packaging materials and 332 types of medical supplies, as well as raise industrial standards for 500 types medical devices.
The Ministry of Health will also re-assess all drugs on the market and crack down on illegal distribution of pharmaceuticals, such as selling medicine online.
According to industry insiders, the plan will benefit large pharmaceutical companies with high-quality products and strong technological capability. It will adversely affect small businesses engaged in price wars. This may result in more mergers and acquisitions in the industry in the next four years, the commission said.

 Relief reaches isolated village
Relief reaches isolated village
 Rainfall poses new threats to quake-hit region
Rainfall poses new threats to quake-hit region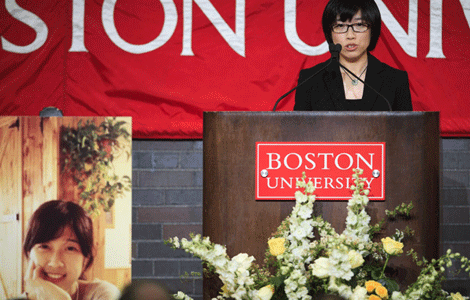
 Funerals begin for Boston bombing victims
Funerals begin for Boston bombing victims
 Quake takeaway from China's Air Force
Quake takeaway from China's Air Force
 Obama celebrates young inventors at science fair
Obama celebrates young inventors at science fair
 Earth Day marked around the world
Earth Day marked around the world
 Volunteer team helping students find sense of normalcy
Volunteer team helping students find sense of normalcy
 Ethnic groups quick to join rescue efforts
Ethnic groups quick to join rescue efforts